Are Aquatic And Costal Systems Denialated More Clearly By Their Animal Life?
Unless otherwise stated, copyright © 2022 The Open up University, all rights reserved.
Printable page generated Wednesday, fifteen Jun 2022, 17:57
Effects of pollutants on the aquatic environment
Introduction
Nosotros can all relate to h2o. Nosotros know nosotros need it to survive – indeed, the early on great civilisations of Arab republic of egypt and Mesopotamia were centred on river valleys where in that location was a plentiful supply of fresh, clean water.
When we take water into our bodies, it is used in several means. For example:
- for cooling – it helps keep our bodies at around 37 °C
- as a waste disposal medium
- as a conductor for nerve impulses
- as a component in the digestion of food
- as a solvent in which vital chemic reactions take identify.
You can see from the above that even if you lot didn't motion an inch, your body would nonetheless need water to keep you alive.
Water is a fascinating subject, encompassing chemistry, biological science and physics. Apart from keeping us alive, water is used extensively in industrial processes, for recreation and for transport. Information technology is something we can't do without.
The water nosotros use for domestic purposes ought to be free from contaminants, however water pollution is a major problem in many countries. According to the Globe Wellness Organisation (WHO, 2002), about ane.7 million people die each year due to unsafe water, sanitation and hygiene. In this text we consider in outline the major sources of pollution and the result that pollutants have on the aquatic surround.
The activities located throughout the text volition help you to review and retrieve what you have read.
This OpenLearn course is an adjusted excerpt from the Open University grade T868 Environmental monitoring and protection.
Learning outcomes
After studying this course, you lot should be able to:
-
listing the major sources of water pollutants;
-
describe the effects on h2o due to organic materials, found nutrients and toxic, concrete and biological pollutants;
-
list the main diseases caused past microorganisms that tin can be carried by water.
ane The hydrological cycle
The hydrological bicycle – the continuous cycling of water between country, open up water surfaces and the sea, either directly or indirectly – is a complex process that has been known about for a long time (Figure one). Probably the oldest reference to the hydrological cycle is plant in the Chandogya, i of the principal Upanishads, which says 'rivers … lead from sea to sea'. It reveals that every bit early as 1000 BCE, attempts were existence made to interpret and explain recurrent phenomena on the basis of straight feel.

Effigy one Early understanding of the water wheel?
The identifiable mechanisms of the bicycle are complicated non only by the characteristics of air–water–state interfaces across which the cycle operates, but also past climatic factors that vary in both time and space. The various operations and mechanisms within the cycle are illustrated in Figure two.
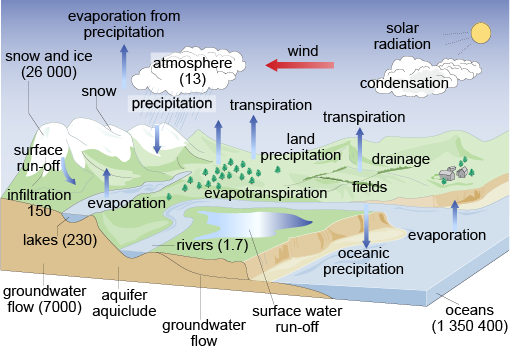
Figure two The hydrological wheel (volumes are in Tmiii = ten12 m3)
2 Sources of pollution
To some extent a river is a self-renewing resource. If polluting discharges to a river are intermittent, the river is often able to return to a clean and unpolluted condition equally the pollutants are flushed out and carried down to the bounding main. In improver, because of the organisms present (eastward.g. bacteria capable of breaking down organic matter), river water has some capacity for self-purification – unless too many of these organisms are killed off too quickly.
Some pollutants are objectionable considering they overload the self-purification processes of the river. Equally rivers are often the raw h2o sources for potable supplies, this tin can accept dire consequences.
An case of such pollution is the discharge of domestic sewage effluent to rivers. In modest quantities it does no serious impairment and may indeed exist beneficial, providing a source of organic carbon that provides nutrients to the animals in the river. But if inadequately treated or in excessive quantities, sewage effluent can seriously damage the constitute and fauna life of a river past reducing the oxygen content of the h2o. In extreme cases, where the oxygen content is reduced to zero (or almost then), the river will support very little life, and volition get foul smelling and grossly offensive. A river in such a state is obviously not desirable as a water source for drinkable supply.
Some industrial effluents discharged in big quantities can be similarly harmful. For case, effluents from the food industry are not particularly toxic, but considering of their organic content and big volume, they can exert a considerable oxygen need on the environment in the region of the discharges.
The two pollution sources described above are classified as betoken sources, as the pollutants are generally collected by a network of pipes or channels and conveyed to a unmarried point of belch. Non-indicate or lengthened sources are characterised by multiple discharge sources that cannot be pinpointed. An example of a diffuse source is run-off from fields and roads.
Point sources are hands controlled, just diffuse sources are about incommunicable to collect and control. The latter pose great challenges in efforts to upgrade the quality of rivers.

Figure 3 A eutrophic lake in southern California
Lakes are much more vulnerable than rivers to pollution. In one case a pollutant enters a lake information technology will stay for a long time. The flushing issue that characterises rivers is much less evident in lakes, and the dilution factor is much less than is available in the bounding main. Merely the self-purifying ability of the water will abate the pollution in the long term. Lakes are thus particularly prone to eutrophication (Figure 3).
Since river pollutants can be controlled more hands at source, it is useful to know where they originate. A list of specific sources would exist very long, but the post-obit categories can be identified:
- discharges from sewage works, which often comprise some industrial wastes
- discharges from manufacturing and industrial plants, including mines
- discharges from animate being rearing, fish farming and agriculture
- seepage from domestic and industrial landfill sites
- urban surface h2o run-off.
Problems occur when the natural characteristics of a river are contradistinct past pollutant discharges. We at present consider the effects of the following categories of pollutants:
- organic materials
- plant nutrients
- toxic pollutants
- physical pollutants
- biological pollutants.
Delight be aware that this categorisation is not absolute. For instance, toxic pollutants may well exist organic, too.
3 Organic materials
Organic substances plant the major freshwater pollutants, coming from domestic sewage discharges (even later handling) and from certain industries such every bit food processing. This section volition bargain with the biodegradable forms, but there are also inert (not-biodegradable) toxic forms. Organic substances can be natural (in which example they are commonly biodegradable) or constructed (in which example they can often be degraded by microorganisms that have adapted to utilising them).
The major polluting effect of biodegradable organic materials is the reduction in oxygen concentration in the water. Bacteria and other organisms (decomposers) break these materials downward into simpler organic or inorganic substances. They employ up oxygen in the procedure, and as their population increases in that location is an extra need for dissolved oxygen.
When a potentially polluting effluent is released into a stream, at that place follows a sequence of events in fourth dimension and distance. This sequence leads to different environmental consequences and different aquatic communities compared with those immediately upstream and the successive reaches downstream. After a certain distance, natural biodegradative processes will break down the pollutants, often returning the river to something like its original status.
Three stages of organic pollution can exist defined.
- When the load is modest, there will be lilliputian alter in the species of plants and animals present in the h2o and fiddling variation in the natural cycles. Initially, the dissolved oxygen will be near to saturation level. Any organic pollution credible at the point of discharge will disappear within a short distance downstream as it is removed by the natural processes of cocky-purification. It could be said that, in some instances, mild organic pollution is beneficial to the river, since it increases the nutrient supply for microorganisms present in the natural land. This minimal pollution tin benefit the whole aquatic ecosystem.
- If the load increases, the dissolved oxygen level will drib significantly and the river volition be polluted for a considerable altitude from the point of discharge. Some species of animals and plants will flourish at the expense of others. In the absence of further pollution, the river will probably recover downstream, but if the area around the discharge remains polluted then this can act every bit a barrier to the passage of migratory fish (amid other disadvantages).
- If the polluting load is increased still further, the natural ecosystem volition be grossly distorted and its effectiveness in coping with the pollutant load greatly reduced. The level of dissolved oxygen will be very low or fall to zero. Often the only organisms to flourish volition be sewage mucus, sure worms and fly larvae. (These organisms give a depression Biological Monitoring Working Party (BMWP) score, indicating the polluted nature of the river.) Also, anaerobic bacteria may thrive and give a foul smell to the h2o by metabolising organic substances and producing methane, hydrogen sulfide and ammonia. Few algae are able to thrive under severe organic pollution, and so reoxygenation by photosynthesis will be hindered. The river will now remain polluted for a much greater altitude downstream.
The sequence of events following significant pollution of a waterway past organic cloth is shown in Figure 4.
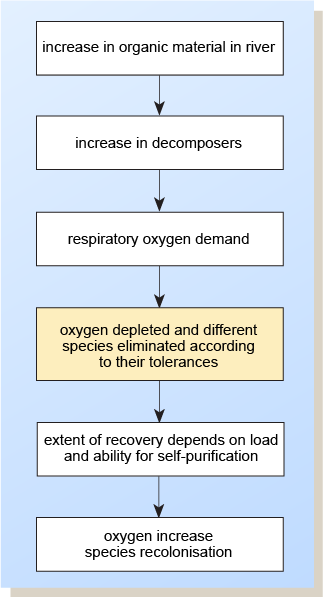
Effigy 4 Events post-obit organic pollution
Inorganic materials tin also cause deoxygenation, e.one thousand. when ferrous fe from mine drainage h2o enters a river (Stumm and Lee, 1961). In the reduced ferrous (Fe(Ii)) land the iron is in solution, but on meeting the oxygen in the river it is oxidised to blood-red insoluble ferric (Fe(III)) iron, a procedure that reduces the concentration of dissolved oxygen in the river water. The oxidised iron is now in intermission so that, as well equally reducing the oxygen content, information technology reduces calorie-free penetration. It finally settles out slowly downstream of the discharge point, giving ascension to all the bug associated with suspended solids. This type of trouble is usually associated with coal-mining effluents.
Action i
Identify which of the following statements concerning the effects of both organic pollution and eutrophication are true. If a statement is false, give the reason why.
a.
a. Depletion of oxygen occurs because of an increase in the activities of primary producers in both cases.
b.
b. In both cases information technology is the presence of plant nutrients (nitrates and phosphates) that causes the death of light-green plants and depletion of oxygen.
c.
c. The departure in the proportion of producers and consumers between organically polluted waters and eutrophic waters is negligible.
d.
d. A reduction of dissolved oxygen in both cases causes the depletion of species.
eastward.
e. Once the dissolved oxygen content is decreased, merely the removal of the offending pollutant can let an increment in species.
The right answers are d and e.
Reply
Statements (d) and (e) are true.
Statement (a) is faux: depletion of oxygen occurs in both cases due to the activities of decomposers. An increase in primary producers (plants) would increase oxygen levels.
Argument (b) is simulated: just in eutrophication are plant nutrients present, and these will usually encourage plant growth.
Statement (c) is false: in organically polluted waters the producers are only a small proportion of the ecology, making up less than 25% of the population, whilst in eutrophic waters they plant more 75%. The proportion of consumers, on the other hand, is similar in both situations (though slightly greater in organically polluted waters).
four Plant nutrients
Certain inorganic substances are essential for normal constitute metabolism merely they tin reach such levels as to be considered pollutants.
Eutrophication is the increment with time of plant nutrients and biota in a watercourse. Farming contributes fifty–60% of nitrates and 20–thirty% of phosphorus getting into UK waters (GOV.UK, due north.d.), with nearly of the remainder coming from treated sewage effluent. Pollutants from these sources tin can greatly accelerate the natural process by increasing the inputs of nutrients and so that algae grow rapidly and algal blooms may course (Effigy 5). The resulting rapid removal of carbon dioxide by photosynthesis can upset the bicarbonate–carbonate equilibrium and cause a pH rise, which in itself is damaging to the ecosystem.
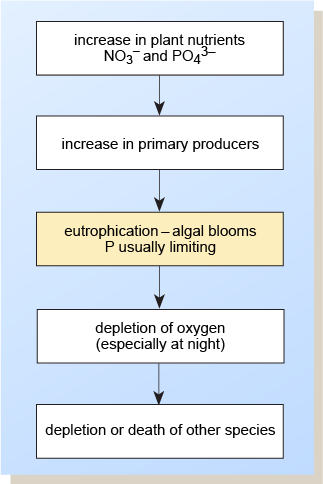
Effigy v Events following eutrophication
In addition, at night the backlog respiration (without the replacement of oxygen by photosynthesis) can deplete oxygen reserves, causing the decease of higher organisms such as invertebrates and fish. This process can be compounded when algal blooms, through their disuse, further reduce the oxygen content of the water.
In shallow h2o, the germination of benthic (bottom-living) mats of algae can create a smothering layer over sediments. This tin can hinder the supply of oxygenated water to eggs and impede the emergence of fry from salmon spawning grounds, for case.
Of the plant nutrients, the total inflow of phosphorus and nitrogen (especially at the productive time of twelvemonth, spring and summer) is the near important. The main types of organism associated with algal blooms, and the dissimilar weather condition needed for their connected growth, are as follows.
-
Blue-green algae (cyanobacteria)
Blue-green algae are able to set atmospheric nitrogen and therefore are non limited by nitrate levels in the water. Neither are they dependent on dissolved carbon dioxide, because they can use the bicarbonate ions nowadays. In add-on, they are tolerant of relatively high pH. Their growth is limited by the phosphorus content.
-
Unicellular green algae
Green algae require nitrate, equally they are unable to comport out nitrogen fixation. They likewise require fairly high levels of carbon dioxide, as they cannot use bicarbonate ions, and they are non tolerant of high pH values. Their growth is limited by both phosphorus and nitrogen.
Contact with or ingestion of blue-dark-green algae can cause skin rashes, eye irritation, airsickness, fever, and pain in the muscles and joints, due to toxins produced by the algae. In addition, 'reddish tides' are harmful algal blooms that announced in coastal areas. They tin produce toxic furnishings in humans, marine organisms and birds. The toxins produced may also make the surrounding air difficult to breathe (NOAA, 2013), and the bloom of algae oftentimes turns the water red.
Generally, increases in both phosphate and nitrate concentrations seem to be the well-nigh important factor in governing the charge per unit of eutrophication in nearly waters. All the same, whether this is the just factor that limits the charge per unit of eutrophication is withal a thing of controversy. Whatever eutrophication is contingent on other aspects of the particular ecosystem such equally the hardness, the pH value and the original distribution of algal species.
For freshwater plants, almost eight times more nitrogen is required than phosphorus. Phosphorus thus limits eutrophication if nitrogen is more than eight times as arable as phosphorus, while nitrogen limits eutrophication if its concentration is less than viii times that of phosphorus (UNEP, n.d.).
Nitrates tin accept a more significant consequence on homo health than phosphates if they are nowadays in drinking water supplies.
- When nitrates are ingested by infants under 6 months of age, they tin be converted to nitrite by bacteria in the digestive organisation. Nitrites combine with haemoglobin in the bloodstream, preventing information technology from conveying out its normal function of combining with oxygen and carrying it around the body. This can result in a serious, though rare, condition called methaemoglobinaemia ('blue babe syndrome'), which tin can be fatal (Skipton and Hay, 1998).
- Ingestion of nitrate and nitrite by people with a low intake of vitamin C increases the run a risk of stomach cancer (Ward et al., 2011).
Water quality standards exist to control pollution of domestic water supplies. The maximum limit prepare by the European union for nitrates in drinking water is 50 g k−3 (as NO3 −) (EU, 1998). Water with a high nitrate content tin be treated for drinking using reverse osmosis.
The effects on the biota of organic pollution and artificial eutrophication are summarised in Figure half-dozen. When organic pollution occurs, the chief types of organisms present are the decomposers. In the case of eutrophication, the producers are dominant, and in larger quantities than in 'make clean' waters.
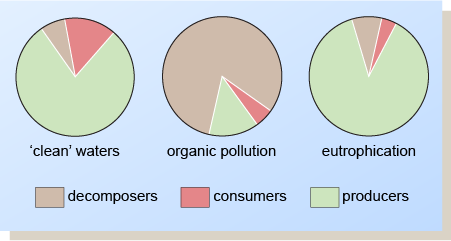
Figure 6 Ecological imbalances caused by organic and inorganic pollutants
five Toxic pollutants
The term 'toxic' is a rather misused one. It is misleading to refer to ane material as a toxic substance and to another as not-toxic without qualification. The toxicity of all materials depends on their concentration. Farther complications may be introduced by the fact that some materials (e.grand. selenium) are essential components of an animal's diet, yet in anything other than very low concentrations they may have a toxic event.
Other ecology factors to be taken into account include:
- the extent of biodegradation
- the rate of aggregating of a substance in the biota (and then inside the nutrient chain)
- the retentiveness time of a substance within an organism.
An important mechanism for toxicity in the trunk is the poisoning of enzymes, which are the catalysts of all the actual functions.
The terms used when explaining the effects of a toxic substance on an organism are:
- lethal – causing decease by directly poisoning
- sublethal – not sufficient to cause death, but leading to a reduction in the number of species and/or individuals by, for instance, causing a change in behaviour, growth or reproductive success
- acute – causing an effect (possibly death) within a short period of time
- chronic – causing an effect (lethal or sublethal) over a prolonged period of fourth dimension
- accumulative – having an effect that is increased by successive doses.
To test the effects of toxicity, the LD50 (lethal dose) test is commonly used. The LD50 is the dose that is large enough to kill 50% of the sample of animals under test. Some examples of LD50 values for different chemicals are given in Tabular array i.
| LDl (mg per kg body weight) | Examples | Nomenclature |
|---|---|---|
| 1–10 | arsenic | highly toxic |
| 10–100 | cadmium copper lead mercury | moderately toxic |
| 100–1000 | aluminium molybdenum zinc | slightly toxic |
| >1000 | sodium iodine calcium potassium | relatively harmless |
Certain inorganic substances, such every bit cyanides, fluorides, sulfides, sulfites and nitrates, may be classified as toxic.
- Compounds of cyanide and sulfide interfere with the use of oxygen in respiratory reactions in cells.
- Excess fluoride can pb to mottling of teeth and bones in humans.
- Nitrates can cause 'blue baby syndrome'.
All the same, the final furnishings of a toxic substance in water depend on environmental factors such as hardness, temperature and pH. Salts of heavy metals such equally copper, silver, lead, gilded, nickel, chromium, zinc, cadmium and mercury are toxic and will generally kill most aquatic organisms at very depression concentrations, merely they are generally less toxic in calcium-rich water (Wilson, 1988); a nickel–cyanide complex is 500 times more toxic to fish at pH 7 than at 8, because the complex dissociates into cyanide and nickel ions and a proportion of the cyanide forms the highly toxic undissociated hydrogen cyanide (HCN); and ammonia is 10 times more toxic at pH 8 than at 7 (EIFAC, 1968). Also an condiment effect, synergism, may occur – for instance, bear witness has been institute for synergism between mercury and uranium (Sánchez et al., 2001).
2 particularly meaning groups of toxic pollutants are the heavy metals, and constructed organic substances such as some of the pesticides.
v.1 Pesticides
Pesticides are chemicals used to impale pests such as weeds, insects, fungi and rodents. Afterwards the Second World War, pesticides such as DDT (dichlorodiphenyltrichloroethane) – which were highly toxic, persistent and bioaccumulating – were normally used in agronomics and for vector control (east.g. confronting the Anopheles mosquito, for malaria command). The populations of birds of prey declined due to eggshell thinning preventing the birth of alive offspring. This was because of DDE (dichlorodiphenyldichloroethylene), a very stable metabolite of Ddt (Faber and Hickey, 1973). Sexual evolution and behaviour in birds such as gulls was also disturbed (Fry and Toone, 1981). The use of Dichloro-diphenyl-trichloroethane has now been banned in nigh countries, and the tendency is to opt for pesticides that degrade chop-chop in the environs (FAO, north.d.).
Chemicals that exhibit the characteristics of Dichloro-diphenyl-trichloroethane (i.e. loftier toxicity, persistence and bioaccumulation) are termed persistent organic pollutants (POPs), and most of the POPs are pesticides. Pesticides have been found to be carcinogenic in experimental animals, and therefore are peradventure carcinogenic to humans. They are suspected of depressing the immune organisation, and of disrupting the endocrine system, mimicking or blocking normal hormone activity (Morner et al., 2002). More than on endocrine disruptors is given beneath.
POPs can be transported by wind (e.g. from combustion and loftier-temperature processes such equally those in the iron and steel industry) and water, and as such tin affect areas far from the point of their use. Their persistence in the environment, and their potential to move up the food chain, led to the Stockholm Convention of 2001, under which nations agreed to reduce or eliminate the production, use and/or release of POPs (Stockholm Convention, 2008).
Organophosphates (such as malathion, diazinon and chlorpyrifos) are insecticides containing phosphorus. They human action by inhibiting enzymes in the nervous systems of animals. Pyrethroids (synthetic versions of the short-lived natural pesticide pyrethrin, which is fabricated from chrysanthemum flowers) are another category of insecticide, often used by householders to control pests such every bit leaf-eating insects and ants. Organophosphates and pyrethroids can attach to soil particles and get done into rivers and streams, endangering aquatic life.
Amid the POPs are polychlorinated biphenyls (PCBs), which are a grouping of organic chemicals used in a variety of ways (east.g. every bit hydraulic fluids, plasticisers, fire retardants, rut transfer fluids, paint additives, lubricants and cutting oils). Ingestion of water containing PCBs can atomic number 82 to an increased risk of cancer, immune deficiency, and bug with the reproductive and nervous systems (EPA, 2012). Their use was banned in 1977.
The main problem with manufactured substances such as pesticides is that most are unknown in nature and thus organisms have not evolved to deal with many of them. Although some will be broken down into harmless substances past normal digestion processes, others will remain and accumulate in the organism exposed to them. This is known every bit bioaccumulation. If this organism falls casualty to some other organism, the toxic substances volition be passed up the food chain and retained in increasing quantities within the bodies of organisms college up the chain. The accumulation of toxic substances through the food chain is chosen biomagnification. An instance of this was the case of Minamata disease, where consumption of fish contaminated by methyl mercury led to thousands of people suffering symptoms such every bit numbness in fingers and lips, difficulty in speech and hearing, inability to command their limbs, and seizures. There were many deaths, too (Porteous, 2008).
v.1.ane Endocrine disruptors
As mentioned in a higher place, pesticides can exist endocrine disruptors. Many other chemicals are likewise classed every bit endocrine disruptors, i.due east. they interfere with the synthesis, secretion, send and binding, action, or elimination of natural hormones in the torso that are responsible for the maintenance of homeostasis, reproduction, development and/or behaviour (Burkhardt-Holm, 2010).
Most endocrine disruptors are synthetic compounds (e.g. plasticisers such every bit bisphenol A, used in plastic bottles and food containers; sex steroids in contraceptive pills; paints; pesticides; alkylphenol polyethoxylates used as surfactants in detergents). They can end upwards in watercourses through sewage treatment works, surface water run-off, direct discharge or leachates from landfill sites. One effect that has been observed on wildlife is the feminisation of male fish (Jobling et al., 2002).
Having said the to a higher place, in that location are also natural sources of endocrine disruptors. For case, Fusarium fungus infesting corn and other grains produces zearalenone, a potent oestrogenic chemical that causes cessation of lactation and hyperoestrogenisation in pigs (Burkhardt-Holm, 2010).
In terms of water supply, endocrine disruptors tin be present if untreated groundwater is used for potable supplies, if the groundwater is contaminated with the doubtable chemicals. Bottled h2o tin incorporate endocrine disruptors from plasticisers and detergents used in the product process.
five.2 Acidity and heavy metals
Acidity tin can exist detrimental to life forms. Table two shows the effects of low pH on fish.
| pH range | Effect |
|---|---|
| vi.v–9.0 | No consequence |
| vi.0–6.4 | Unlikely to exist harmful except when carbon dioxide levels are very loftier (1000 mg l−i) |
| 5.0–5.9 | Not peculiarly harmful except when carbon dioxide levels are high (20 mg l−i) or ferric ions are present |
| 4.5–4.ix | Harmful to the eggs of salmon and trout species (salmonids) and to adult fish when levels of calcium, sodium and chloride are low |
| 4.0–four.4 | Harmful to adult fish of many types that have not been progressively acclimated to low pH |
| iii.v–three.nine | Lethal to salmonids, although acclimated roach can survive for longer |
| 3.0–3.4 | Most fish are killed within hours at these levels |
(EPA, 2000)
In addition, highly acidic waters tin can dissolve heavy metals, particularly if the pH is below 3. This occurs in the case of mine wastewaters. The metals present tend to be brought into solution, especially iron, zinc, lead and molybdenum.
The information that is available on metal pollutants tends to refer to the full concentration of the metals; unfortunately this provides fiddling information on a metal'southward bioavailability (i.e. the power of an organism to take up the metal) or how long the metal will stay in solution earlier it is removed into sediment. To be able to predict the adverse furnishings of metal pollutants in h2o it is necessary to decide the physical form or chemical speciation of the metal present, since metals can exist in a broad range of forms. The subject is besides complicated to be covered fully here, but the following two theoretical examples are extreme cases of metal pollution to show the range of events that can occur.
- Instance i: Afterward treatment to remove nigh of the pb, the effluent from a atomic number 82–acid bombardment factory is discharged into a river. The lead concentration in the effluent is even so about 4 g m−3, with a trace of particulate pb in the form of lead sulfate. The lead sulfate settles out quickly. The soluble lead becomes attached to particles greater than 12 µm in size and settles out in the river. The discharge therefore causes a relatively small increment in the pb content of the river.
- Example two: A sewage effluent is contaminated with cadmium from industrial sources. The cadmium is in the form of organic complexes formed from the organic-rich sewage in the sewage handling process. In the river this form of cadmium does not settle out and is carried downstream over a long distance, remaining bachelor to biological life throughout.
Metals dissolved in water tin enter the food chain by a multifariousness of mechanisms. For example:
- Phytoplankton absorb metals by diffusion beyond the external membrane.
-
Fish can take in metals by
- diffusion across the membrane of their gills
- ingesting metals, though metals taken in this way are not as readily bioavailable.
- Filter feeders such as oysters and cockles inhabit the surface of sediments and consume considerable amounts of particulate matter from the h2o that passes through them; they accumulate metals by ingestion.
half dozen Physical pollutants
Physical pollutants include:
- suspended solids
- immiscible liquids
- discharges that consequence in changes to the temperature or flow rate of the receiving h2o
- substances that impart a taste, aroma or colour to the water.
half dozen.1 Suspended solids
Various industries produce suspended textile (or particulates) in their effluents, and this has several consequences.
All solids tend to reduce light penetration, and then the growth of plant life in watercourses is inhibited. This volition have secondary effects on food chains. Lesser-living animals and plants may be smothered every bit particles settle. If the particles settle on gravels, fish spawning tin be seriously disrupted. Predators that hunt by 24-hour interval may be restricted in their activities: for case, in turbid water there may exist an abundance of leeches as fish are no longer able to see and consume them as food.
One of the virtually of import effects on animals is the damage to fish gills. Prolonged exposure to high levels of suspended solids (50 mg l−1 and above) is probable to lead to sublethal changes due to respiratory distress, and agin growth and development (Au et al., 2004).
Some effluents pollute because substances in them enter into a chemical reaction with salts already dissolved in the water. For case, iron hydroxide may exist precipitated if water containing atomic number 26 is discharged into a naturally alkali metal river. This phenomenon typically arises from abandoned mines, where the clear h2o pumped out and discharged into a clear stream can produce a bright orangish coloration that prevents penetration of light and hence inhibits plant life.
Some suspended solids can also cause harmful effects when soluble toxic components nowadays in them are dissolved into the h2o by biological or chemical action.
6.two Immiscible liquids
Immiscible liquids may exist present every bit oils, greases or tarry substances, often in the grade of an emulsion (a colloidal pause of i liquid in another, every bit in mayonnaise). They may impact turbidity in the aforementioned way as suspended solids. All the same, emulsions are not likely to settle to the bed of the river. Frequently they float on the surface and attach to vegetation at the waterline. Some immiscible liquids are decomposed slowly by aquatic microorganisms. Many oils and tars are slightly soluble in h2o and thereby impart tastes and odours to it.
Oil is generally less dumbo than water and will spread over the surface to form an extremely sparse, often visible film; a small quantity of oil is therefore likely to pollute a large surface area. Even when the oxygen demand in the water is low and oil imposes little additional biological load, the presence of an oil picture show with a thickness of only one thousandth of a millimetre (1 µm) may reduce the rate at which oxygen is transferred from air to water. It tin also affect the life cycle of insects, since the larvae of some species bladder on the surface.
Oil is one of the more than serious pollution problems. As an example, there are now around 3000 pollution incidents involving oil and fuels every yr in England and Wales (Environment Agency, 2013). Although some of these bear upon land, the vast bulk bear on the water environment.
6.3 Discharges contributing to a temperature change
Industrial effluents are frequently discharged at temperatures different from those of the receiving river. Almost invariably the effluent is warmer than the river, since h2o is widely used for carrying away heat.
Inside limits, a raised temperature increases the metabolic rates of all aquatic organisms. It also decreases the concentration of dissolved oxygen needed for saturation – for instance, the saturation concentration of oxygen in water at five °C is 12.79 thou m−3, while at 15 °C information technology is 10.01 g 1000−3. The overall upshot on the oxygen balance of a particular heated effluent therefore depends to a certain extent on the oxygen remainder in the river at the point of discharge.
A small-scale increase in the temperature of a clean, fast-flowing stream may not touch the ecosystem adversely. Provided oxygen is plentiful, plant and creature populations may be altered slightly but remain in a counterbalanced land. Species indigenous to warmer climates may become established in a heated portion of a river. Yet, heated effluents are commonly discharged to watercourses that are already polluted to some degree, and then the polluting furnishings are compounded. A heightened biochemical oxygen demand (BOD) on the river water due to a sewage discharge upstream may be exacerbated past raising the temperature. Any animals or plants that die as a result of the oestrus or greater oxygen deficit are decomposed by bacteria, which decreases the oxygen level even more than.
half-dozen.iv Discharges causing variations in period rate
Variations in the period of a river can result from excessive abstraction or from intermittent discharges of relatively large volumes of effluent, equally when settling ponds (which are used to remove particulates from effluents in the ceramic industry, for example) are emptied. At that place are, however, maximum limits that must be adhered to.
Since the organisms that become established in a river will be those best suited to its conditions, sudden and repeated fluctuations in the rate of flow will mean that simply those organisms that can withstand the changes will survive. Plants growing in silt deposits on the bed of a stream will be destroyed when the silt is washed away by a sudden increment in menses. When the menses falls, organisms that are dependent on a high dissolved oxygen concentration will die if the river reverts to a serial of near-stagnant pools.
6.5 Substances causing taste, odour and coloration
Very low concentrations of some chemic compounds will produce unpleasant tastes and odours, or will taint the mankind of fish living in h2o contaminated by them. Interaction betwixt substances may produce tastes that are credible at concentrations well beneath those at which either substance is individually detectable. An 'clarified' taste of chlorinated tap water is obvious if the raw water supply contains phenols, since this results in the formation of chlorophenols. (Phenolic compounds tin occur naturally in lowland rivers.) Unpleasant tastes and smells, unremarkably earthy or sulfurous in nature, can too occur naturally from decaying vegetation.
The ecological upshot of colour will depend on its light-absorptive properties in relation to the spectral requirements of algae and plants (i.east. which wavelengths of light they need). Many rivers are naturally coloured (e.g. those draining peat are low-cal brown due to humic and fulvic acids) and still are able to back up biota, including trout. The ecological effects of colour are unremarkably minimal compared with other factors.
Acivity two
Each 60 minutes, an industrial plant discharges 600 m3 of treated effluent at 40 °C into a river that has an annual average temperature of xv °C. The flow rate of the river is a constant 20 000 g3 per twenty-four hours.
- a.Assuming perfect mixing, what will the temperature in the river be after entry of the effluent, assuming no temperature loss to atmosphere?
- b.What bear on will this accept on the river?
Reply
-
a.The effluent flow rate is 600 mthree per hour, which equates to 14 400 m3 per solar day. The final temperature in the river will be:
-
b.The higher temperature will:
- increase the metabolic rate of the aquatic organisms in the river
- decrease the level of dissolved oxygen, affecting organisms and plants in the water
- cause species more suited to waters warmer than the ambient to become established
- preclude fish from migrating upstream past the entry point of the heated effluent.
7 Biological pollutants
Biological pollutants are organisms that may be harmful to other forms of life, only they have to be ingested to take any effect. The about usual form of transmission is the faecal–oral road, in which faecal matter from one human existence finds its style into another. This can happen through the ingestion of faecally contaminated h2o or food. Alternatively, sometimes pathogenic organisms tin exist consumed through eating contaminated undercooked food, as in the case of certain strains of Escherichia coli.
The effects of the different organisms are varied, and can be more easily ascertained through sources such every bit the WHO or local health protection agencies. However, in this subsection I will describe the characteristics of the main h2o-borne pollutants, namely:
- pathogenic bacteria
- coliforms
- faecal streptococci
- Clostridium perfringens
- viruses
- protozoa
- helminths
- other biological pollutants.
7.1 Pathogenic bacteria
As well every bit the leaner that are constitute naturally in river water and that are essential for the natural cycle of nutrients, at that place may exist other, less desirable bacteria. Pathogenic bacteria (such every bit Salmonella typhi, Figure 7) can crusade disease in a variety of organisms, including humans. Since the presence of pathogenic bacteria is generally due to the activities of humans, information technology constitutes a form of pollution. Non-pathogenic leaner, past definition, are harmless; indeed, as already mentioned, they can exist beneficial and form an essential part of the aquatic ecosystem.
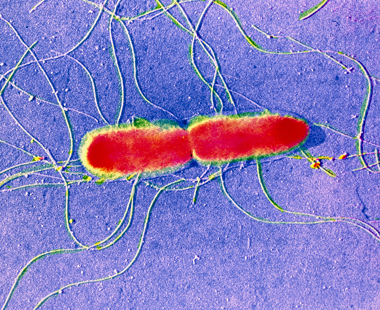
©
Copyright © CNRI/Science Photo Library
Figure 7Salmonella typhi
Effluents from sewage treatment works contain some pathogenic bacteria, simply in far smaller numbers than in incoming sewage since the sewage treatment processes volition generally eliminate more than 99% of them.
Since pathogenic bacteria are accustomed to human body temperature (about 37 °C), they do not flourish in river h2o and dice off relatively quickly.
7.2 Coliforms
Coliforms are a large group of bacteria, oftentimes of intestinal origin. The coliform Escherichia coli (Figure 8) is present in the intestines of humans and other mammals. Its presence in water implies that man pathogens from faeces may also be nowadays, and it is therefore a useful indicator organism of faecal contamination.
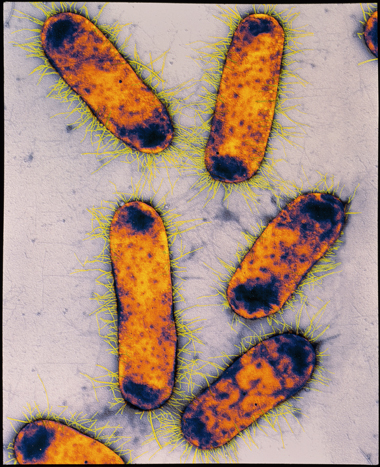
©
Copyright © Middle of Science/Science Photo Library
Figure 8Escherichia coli
E. coli is a rod-shaped faecal coliform about 0.five μm by two–iii μm in size. The strain of E. coli commonly nowadays in humans is harmless and occurs consistently in faeces in far greater numbers than pathogenic bacteria. Yet, there are other strains – such as E. coli O157:H7 – that are pathogenic. These have been found in partially cooked meat and take led to deaths (Rangel et al., 2004).
Concentrations of E. coli equally low every bit 10 cells per litre tin exist detected. The presence or absenteeism of E. coli in a h2o sample provides an of import indicator of pollution and possible hazard to public health.
7.iii Faecal streptococci
The faecal streptococci group of bacteria consists of the species Streptococcus faecalis (Figure nine), S. faecium, South. durans, South. equinus and South. bovis. The bacteria are approximately ane µm in bore and occur in bondage of varying length. They die fairly apace outside their host, and so their presence is indicative of recent pollution.
©
Copyright © CNRI/Science Photo Library
Figure 9Streptococcus faecalis
7.4 Clostridium perfringens
C. perfringens (Figure x) is an anaerobic organism present in the intestines of humans and animals at much lower numbers than E. coli. Information technology is a mutual crusade of food poisoning. The cells are rod-shaped (about 5 µm by one µm) and can form endospores. They tin can cause severe intestinal balk and diarrhoea if present in water that is ingested.
©
Copyright © CNRI/Science Photo Library
Effigy xClostridium perfringens
Endospores – or spores, as they are unremarkably referred to – are hardy structures that certain leaner can form when their environment becomes unfavourable for growth. The purpose of the endospore is survival; information technology is very resistant to estrus and desiccation, and may survive for many years at normal temperatures. When environmental conditions are favourable for growth, the endospore reactivates to form a normal, unmarried cell. This power leads to C. perfringens being present in unchanged numbers long later on other faecal indicators have died out. Thus its presence in the absence of Eastward. coli indicates intermittent faecal contamination.
7.5 Viruses
Viruses are tiny (5–30 nanometres in size) infective agents that tin grow simply in living cells. The virus that causes smallpox, declared eradicated from the world in 1979, is illustrated in Figure xi.
©
Copyright © Heart of Science/Science Photo Library
Figure 11 Smallpox viruses (Variola)
Virtually viruses are able to remain viable in water at depression temperatures, provided in that location is some organic thing present. Once excreted, the number of viruses cannot increase since they simply multiply inside living susceptible cells.
The main threat to water quality comes from human enteric (intestinal) viruses that are produced by infected persons and excreted faecally. Depending on local circumstances, this may contaminate river water straight or, if treated, via sewage effluent that may be discharged into a river. If the river water is abstracted and treated for drinking purposes, the viruses may non be completely removed. (It is possible for a person to be susceptible to only one viral particle.)
The presence of any enteric virus can exist taken as an indication of the possible presence of other harmful viruses. In temperate climates, enteric viruses occur at peak levels in sewage during late summer and early autumn. The exception is the hepatitis virus, which increases in the colder months.
seven.6 Protozoa
There have been several outbreaks of protozoal infections from water in several countries. For instance, each yr in the UK there are 3000–6000 confirmed cases of cryptosporidiosis, caused by the protozoa Cryptosporidium; the largest outbreak was in Torbay, Devon in 1995, when 575 people were taken sick (Hunter et al., 2003).
The most common symptom of cryptosporidiosis is watery diarrhoea. Other symptoms include stomach pain, nausea, vomiting, fever and weight loss (Centers for Disease Control and Prevention, 2010). In addition, some people can act as carriers of Cryptosporidium.
Some other, similar condition is giardiasis, acquired by the protozoa Giardia. Giardiasis outbreaks are non common in the Uk, just one occurred in Bristol in 1985, when 108 cases were diagnosed (Jephcott et al., 1986).
Techniques for sampling and analysis of Cryptosporidium are complicated and time-consuming, requiring the filtration of large volumes of water (100–m litres), followed by several stages of elution, isolation and concentration of the oocysts, and then identification and enumeration by immunofluorescent microscopy. Initial testing does not provide information on whether the oocysts are viable and therefore capable of causing disease – this requires farther testing. Equally a result, there is no specific standard for the organism in EU or UK regulations. In that location is, however, a general requirement that drinking water should not contain any microorganism or parasite at a concentration that would constitute a potential danger to human health (Water Great britain, 2011).
Cryptosporidium and Giardia can be trapped past membrane filtration or slow sand filters. Other types of filters, such as wound fibre filters, are likewise employed. Chlorination and UV radiations at normal doses are ineffective confronting these organisms.
7.7 Helminths
Helminths (Figure 12) are parasitic worms that can cause sick wellness in humans. They range from a millimetre long to more than than a metre (Baron, 1996).
©
Copyright © RGB Ventures LLC dba SuperStock/Alamy
Figure 12 A helminth, Ascaris lumbricoides
Helminths tin can cause morbidity, and sometimes death, by compromising the nutritional status of the infected person. They can besides bear on cognitive processes, induce tissue reactions and provoke intestinal obstacle or rectal prolapse (WHO, 2013).
Infection occurs through ingestion of helminth eggs that are present in food. For instance, helminth eggs may be nowadays in the meat of cattle grazing on country that is contaminated by poorly treated sewage effluent or sludge.
7.8 Other biological pollutants
Many other forms of pollution mentioned previously could be considered every bit biological pollutants, e.m. algal blooms and the growth of sewage mucus. Examples that are more clearly of this type of pollution are the many species of blue-dark-green algae, which produce substances that are toxic to terrestrial organisms and can impart tastes and odours to water.
Activity 3
Identify which of the post-obit statements are truthful. If a argument is false, requite the reason why.
a.
a. Continuously flowing organic pollutants such as domestic sewage cannot cause long-term damage to a watercourse in the way that toxic pollutants can.
b.
b. A temperature rise in a waterway leads to higher productivity but doesn't affect the BOD.
c.
c. Effluents from fish farms can be badly polluted. Concern has arisen virtually these effluents mainly because they incorporate unconsumed food and faecal matter from fish.
d.
d. Pesticide pollution of watercourses is due solely to large-scale use of these compounds by farmers.
e.
e. Metals that are h2o soluble are able to bioaccumulate past diffusing across the biological membranes of organisms.
The correct answers are c and e.
Reply
Statement (e) is true. Statement (c) is also true, though business organization has likewise arisen because fish farm effluents incorporate antibiotics (see Department ii).
Statement (a) is false: heavy organic loads on a watercourse can cause long-term harm to the water in the vicinity of the effluent outfall. However, recovery is more likely if pollution ceases than would be the case for toxic chemicals.
Statement (b) is false: the BOD will increment, equally the metabolic rates of the microorganisms will be higher at a college temperature.
Statement (d) is simulated: householders use a large quantity of pesticides in the form of herbicides and insecticides, and these can besides contribute to water pollution.
Activity 4
Identify which of the following statements are true. If a argument is false, give the reason why.
a.
a. The toxicities of private chemicals in a mixture tin can be summed up to give an overall toxicity figure. This is simpler than testing a circuitous mixture.
b.
b. In toxicity tests for a proposed discharge, the near sensitive species in the receiving watercourse should exist used as the test organism.
c.
c. When suspended solids settle on the river bed, it is only benthic plants that are afflicted, as the light volition not achieve the leaves.
d.
d.E. coli is a species of bacterium that inhabits man intestines and is always harmless.
e.
e.Clostridium perfringens is a pathogenic bacterium that lives in the human being gut and can cause food poisoning.
f.
f. Controlling the belch of the culprit chemicals from industry will overcome the problem of endocrine disruptors.
The correct answers are b and e.
Answer
Statements (b) and (e) are true.
Argument (a) is false: there may exist synergistic effects between the individual chemicals in the mixture.
Statement (c) is false: fish spawning and the environmental of invertebrates are also disrupted.
Argument (d) is false: certain strains of Eastward. coli (east.thousand. E. coli O157:H7) are pathogens and can cause fatalities.
Statement (f) is fake: the discharge of oestrogens, often at high levels, due to the use of contraceptive pills will however contribute to the trouble.
Activeness 5
Signal the causative amanuensis (virus, bacterium, protozoan or helminth) of each of the following diseases:
- cholera
- poliomyelitis
- typhoid
- bilharzia
- anthrax
- cryptosporidiosis.
Reply
| Cholera | bacterium |
| Poliomyelitis | virus |
| Typhoid | bacterium |
| Bilharzia | helminth |
| Anthrax | bacterium |
| Cryptosporidiosis | protozoan |
7.9 Typical pathogens
Table 3 lists some of the major pathogens that cause affliction to humans and are likely to be found in polluted water.
| Organism blazon | Organism | Illness | Remarks |
|---|---|---|---|
| Bacteria | Vibrio cholerae | Cholera | Transmitted via sewage and polluted waters in cholera-endemic areas |
| Salmonella typhi | Typhoid fever | Common in sewage | |
| Salmonella paratyphi | Paratyphoid fever | Common in sewage | |
| Salmonella spp. | Food poisoning | Cause of nutrient poisoning; usually institute in contaminated nutrient of fauna origin | |
| Shigella spp. | Bacillary dysentery | Polluted waters are chief source of infection | |
| Bacillus anthracis | Anthrax | Tin can be constitute in effluents from tanneries processing hides from infected animals; spores resistant to treatment | |
| Brucella spp. | Brucellosis (Malta fever) in humans; contagious ballgame in sheep, goats and cattle | Normally transmitted by infected milk or past contact | |
| Leptospira icterohaemorrhagiae | Leptospirosis (Weil's illness) | Carried by sewer rats; also present in water contaminated by urine from infected animals and humans; tin infect through cuts in skin, or intact skin if immersed for a long fourth dimension | |
| Viruses | Poliovirus | Poliomyelitis | Transmitted by faecal–oral road via contaminated food or water |
| Hepatitis A virus (HAV) | Hepatitis A | Transmitted past faecal–oral route via contaminated food or water | |
| Protozoa | Entamoeba histolytica | Amoebic dysentery | Spread by contaminated waters and sludge used as fertiliser; common in warm countries |
| Giardia lamblia | Giardiasis | Found in inadequately treated water | |
| Cryptosporidium spp. | Cryptosporidiosis | Carried by agronomical livestock and infected persons | |
| Helminths | Taenia saginata | Tapeworms | Eggs very resistant, present in sewage sludge and sewage effluents; can be nowadays in contaminated water sources and food |
| Ascaris lumbricoides | Nematode worms | Nowadays in sewage effluents and stale sludge used as fertiliser | |
| Schistosoma haematobium, Schistosoma mansoni | Bilharzia | Carried past water snails in rivers and irrigation ditches contaminated by human waste in specific regions of the world; enter humans by direct penetration of skin |
Conclusion
H2o is crucial for our survival. Information technology is used in our bodies for a number of purposes:
- cooling
- as a waste product disposal medium
- as a conductor for nervus impulses
- equally a component in the digestion of food
- every bit a solvent in which chemical reactions take place.
The hydrological cycle is the continuous cycling of water betwixt country, water surfaces and the sea. Pollutants entering a river can be washed away to body of water, or degraded by microorganisms present in the river. Excess pollution in a river can impairment the found and animate being life present in the river by reducing the oxygen content of the h2o.
Point sources of pollution are discharge points where pollutants collected past a network of pipes or channels are released. Diffuse sources, on the other hand, are characterised by multiple discharge points that cannot exist located exactly. Bespeak sources tin be easily controlled, while diffuse sources pose bully difficulty in terms of drove and command.
Lakes are much more decumbent than rivers to pollution as they do not accept the flushing consequence of rivers. They also do non accept the dilution effect of large bodies of water such every bit the body of water. Eutrophication tin can be a detail trouble for lakes.
The major sources of h2o pollution are:
- discharges from sewage works, oft containing industrial wastes
- discharges from manufacturing and industrial plants, including mines
- discharges from animate being rearing, fish farming and agriculture
- seepage from domestic and industrial landfill sites
- urban surface water run-off.
Different pollutants affect the aquatic environment in different means. While at low concentrations many pollutants (e.k. organic materials, Northward and P) may exist beneficial, at high levels they can adversely impact the ecology of the system. Excess nitrate can be particularly harmful to babies.
Many of the toxic pollutants in effluents are synthetic, and therefore do not hands biodegrade naturally.
The effects of concrete pollution on the ecology of a river system can be circuitous, affecting the feeding and convenance habits of the different species.
Biological pollutants can spread disease through water, and also disrupt the ecology.
The measurement and control of water quality is therefore of crucial importance in the interests of public wellness and the maintenance of the surround.
Table 4 gives a summary of the furnishings of the different pollutants discussed in this unit.
| Pollutant | Full general effect | Effect on biota | Effect on h2o supplies | Sources: natural | Sources: event of homo activity |
|---|---|---|---|---|---|
| Organic (biodegradable wastes) | Increased oxygen need; nutrient provided for organisms lower downwards in food chain | Tolerated in moderate quantities if release not too quick, serious if dissolved oxygen (Do) drops likewise speedily | Increased demand of handling | Run-off and seepage through soil | Domestic sewage, food processing, animal wastes |
| Constitute nutrients | Excessive constitute growth | Need on DO | Increased demand of handling | Natural degradative processes | Animal wastes, fertilisers, detergents, industrial wastes |
| Toxic chemicals (e.g. heavy metals, pesticides, phenols, PCBs) | Toxic to humans, animals and plants | Could exist lethal | Increased demand of treatment or control | Rare | Detergents, pesticides, tanneries, pharmaceuticals, wool scouring, refineries |
| Endocrine disruptors | Alteration of ecology | May adversely touch on health and reproduction of humans and animals | Can be nowadays in water sold in plastic bottles | Fusarium species of fungus | Chemic manufacture, intensive farming |
| Acids/alkalis | Lowering/raising of pH; acids can dissolve heavy metals | Only narrow range of pH tolerable for near plants and animals; heavy metals toxic | Corrosion | Naturally acid or alkali metal rock | Battery, steel, chemic and material manufacturing; coal mining |
| Suspended solids | Reduction in light penetration (increased turbidity), blanketing, introduction of colour | Photosynthesis reduced; blanketing of benthic plants and animals; obstruction of gills of fish | Obstruction of filters; increased need of treatment | Soil erosion, storms, floods | Pulp mills, quarrying, any edifice or development work involving ground disturbance |
| Immiscible liquids | Formation of a layer at the water surface that could forestall O2/CO2 interchange | Reduced Practice; insect breeding affected | Interference with handling processes | Unlikely | Oil-related activity |
| Heat | Decrease in Exercise; increase in metabolic rate of aquatic organisms | Possible reduced breeding or growth of aquatic organisms | None | Unlikely | Power plants, steel mills |
| Gustation-, odour- and colour-forming compounds | Taste, malodour, colour | Tainting of fish | Increased need of treatment | Peat | Chemical manufacture or processing |
| Microorganisms | Pathogenic to humans | None | Increased demand of treatment | Animal excrement | Contamination from human wastes |
Glossary
- algal blooms
- A rapid increment in the population of algae in an aquatic system.
- bioaccumulation
- The accumulation of a substance, such every bit a pesticide or another organic chemical, in the tissues of a living organism.
- bioavailability
- Of a substance, the degree to which or rate at which that substance is absorbed or becomes available at the site of physiological activity, i.e. the extent to which it tin be taken upwards by living organisms.
- biomagnification
- The sequence of processes in an ecosystem by which higher concentrations of a particular chemic are reached in organisms higher up the food chain.
- catalyst
- A substance that increases the charge per unit of a chemic reaction without itself undergoing any permanent chemic change.
- chemical speciation
- The chemical form or compound in which an element occurs in both not-living and living systems.
- colloidal
- Term referring to a organization in which finely divided particles, which are approximately 10 × 10−10 m to 10 000 × 10−10 m in size, are dispersed inside a continuous medium in a manner that prevents them from being filtered easily or settled apace.
- diffuse sources
- Sources of pollution (often minor) that have no specific point of discharge.
- diffusion
- The movement of atoms or molecules from an expanse of high concentration to one of depression concentration.
- dissociates
- In reference to ionic compounds (complexes or salts), to carve up or divide into smaller particles, ions or radicals, usually in a reversible fashion.
- emulsion
- A mixture of ii or more than liquids that are normally immiscible.
- enzyme
- A substance produced past a living organism that acts as a goad to bring nigh a specific biochemical reaction.
- homeostasis
- Maintenance of a constant internal environment.
- hydrological cycle
- The natural water bike that describes the continuous move of water on, above and below the surface of the Globe.
- nitrogen fixation
- The procedure by which nitrogen gas (Northii) in the atmosphere is converted into ammonia (NH3).
- persistent organic pollutants
- (POPs) Organic compounds that are highly toxic, persist in the environs, bioaccumulate in human and fauna tissue, and can be transported by wind and h2o. Most POPs are pesticides.
- point sources
- Unmarried identifiable sources of pollution from which pollutants are discharged, such as a pipe.
- synergism
- Condition in which the interaction of two or more substances results in a combined effect that is greater than the sum of their separate furnishings.
References
Au, D.Westward.T., Pollino, C.A., Wu, R.S.S., Shin, P.G.Due south., Lau, S.T.F. and Tang, J.Y.M. (2004) 'Chronic effects of suspended solids on gill structure, osmoregulation, growth, and triiodothyronine in juvenile green grouper Epinephelus coioides', Marine Ecology Progress Series, vol. 266, pp. 255–64.
Baron, South. (1996) Medical Microbiology, 4th edn, Galveston, University of Texas Medical Branch.
Burkhardt-Holm, P. (2010) 'Endocrine disrupters and h2o quality: a land-of-the-art review', International Periodical of Water Resource Development, vol. 26, no. 3, pp. 477–93.
Centers for Disease Control and Prevention (2010) Parasites – Cryptosporidium (likewise known every bit 'Crypto') [Online], US Government. Available at www.cdc.gov/parasites/crypto/gen_info/infect.html (Accessed v June 2013).
EIFAC (1968) Water Quality Criteria for European Freshwater Fish: Report on Extreme pH Values and Inland Fisheries [Online], Rome, European Inland Fisheries Advisory Commission. Available at www.fao.org/docrep/017/71852e/71852e.pdf (Accessed eleven March 2013).
Surroundings Agency (2013) What is Inland Oil Pollution? Key Issues as the Environment Agency Sees It [Online]. Available at www.environment-agency.gov.uk/research/library/position/41233.aspx (Accessed 6 June 2013).
EPA (2000) 'Generalized short-term effects of acidity on fish', National Water Quality Inventory: 1998 Report to Congress, Ecology Protection Agency, U.s.a..
EPA (2012) Bones Information Most Polychlorinated Biphenyls (PCBs) in Drinking Water [Online], Environmental Protection Agency, Usa. Available at water.epa.gov/drink/contaminants/basicinformation/polychlorinated-biphenyls.cfm (Accessed 6 June 2013).
Eu (1998) 'Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption', Official Journal of the European Communities, Brussels, European Union.
Faber, R.A. and Hickey, J.J. (1973) 'Eggshell thinning, chlorinated hydrocarbons, and mercury in inland aquatic bird eggs, 1969 and 1970', Pesticide Monitoring Periodical, vol. seven, pp. 27–39.
FAO (n.d.) 'Pesticides as h2o pollutants' in Control of Water Pollution from Agronomics [Online], Nutrient and Agriculture Organization of the Un. Available at world wide web.fao.org/docrep/w2598e/w2598e07.htm (Accessed 6 June 2013).
Fry, D.M. and Toone, C.One thousand. (1981) 'DDT-induced feminization of dupe embryos', Scientific discipline, vol. 213, pp. 992–4.
GOV.U.k. (due north.d.) Reducing and Controlling Agricultural Pollution [Online], Crown Copyright. Available at www.gov.uk/regime/policies/improving-water-quality/supporting-pages/reducing-and-controlling-agronomical-pollution (Accessed 15 Apr 2013).
Hunter, P.R., Waite, Chiliad. and Ronchi, Due east. (2003) Drinking Water and Infectious Illness, London, IWA Publishing.
Jephcott, A.Due east., Begg, N.T. and Bakery, I.A. (1986) 'Outbreak of Giardiasis associated with mains water in the Britain', The Lancet, vol. 327, no. 8483, pp. 730–2.
Jobling, S., Coey, S., Whitmore, J.G., Kime, D.Eastward., Van Expect, M.J.Westward., McAllister, B.G., Beresford, N., Henshaw, A.C., Brighty, One thousand., Tyler, C.R. and Sumpter, J.P. (2002) 'Wild intersex roach (Rutilus rutilus) have reduced fertility', Biology of Reproduction, vol. 67, pp. 515–24.
Morner, J., Bos, R. and Fredrix, Thousand. (2002) Reducing and Eliminating the Use of Persistent Organic Pesticides [Online], Geneva, Inter-Organization Plan for the Audio Management of Chemicals. Available at world wide web.unep.org/hazardoussubstances/Portals/ix/Pesticides/POPred_E.pdf (Accessed 6 June 2013).
NOAA (2013) A "red tide" is a common term used for a harmful algal bloom [Online], National Oceanic and Atmospheric Administration, USA. Available at oceanservice.noaa.gov/facts/redtide.html (Accessed 6 June 2013).
Porteous, A. (2008) Dictionary of Environmental Scientific discipline and Technology, quaternary edn, Chichester, John Wiley & Sons Ltd.
Rangel, J.G., Sparling, P.H., Crowe, C., Griffin, P.K. and Swerdlow, D.L. (2004) 'Epidemiology of Escherichia coli O157:H7 outbreaks, The states, 1982–2002', Emerging Infectious Diseases, vol. xi, no. iv [Online]. Available from wwwnc.cdc.gov/eid/article/eleven/4/04-0739.htm (Accessed 5 June 2013).
Sánchez, D.J., Bellés, M., Albina, Thousand.L., Sirvent, J.J. and Domingo, J.L. (2001) 'Nephrotoxicity of simultaneous exposure to mercury and uranium in comparison to individual effects of these metals in rats', Biological Trace Element Inquiry, vol. 84, nos ane–3, pp. 139–54.
Skipton, Due south. and Hay, D. (1998) G98-1369 Drinking H2o: Nitrate and Methemoglobinemia ('Blue Babe' Syndrome) [Online], University of Nebraska – Lincoln. Available at digitalcommons.unl.edu/cgi/viewcontent.cgi?article=2429&context=extensionhist (Accessed 21 Feb 2013).
Stockholm Convention (2008) About the Convention [Online]. Available at chm.pops.int/Convention/tabid/54/Default.aspx (Accessed six June 2013).
Stumm, Westward. and Lee, Thou.F. (1961) 'Oxygenation of ferrous iron', Industrial and Engineering Chemical science, vol. 53, pp. 143–half dozen.
UNEP (northward.d.) 'Where nutrients come up from and how they cause eutrophication' in Water Quality: The Impact of Eutrophication [Online], Lakes and Reservoirs vol. iii, United Nations Environment Programme. Bachelor at www.unep.or.jp/ietc/publications/short_series/lakereservoirs-3/three.asp (Accessed 11 March 2013).
Ward, M.H., Kilfoy, B., Sinha, R., Hollenbeck, A.R., Schatzkin, A. and Cross, A. (2011) 'Ingestion of nitrate and nitrite and risk of stomach cancer in the NIH-AARP Nutrition and Wellness Study', Epidemiology, vol. 22, no. 1, pp. S107–eight.
Water UK (2011) Cryptosporidium [Online]. Available at www.water.org.britain/home/policy/positions/cryptosporidium (Accessed v June 2013).
WHO (2002) The Earth Health Report [Online], World Health System. Bachelor at www.who.int/whr/2002/en/whr02_en.pdf (Accessed 23 January 2013).
WHO (2013) Helminths [Online], Earth Health Organization. Available at www.who.int/tdr/diseases-topics/helminths/en/ (Accessed 6 June 2013).
Wilson, R.S. (1988) 'A survey of the zinc-polluted River Nent (Cumbria) and the East and West Allen (Northumberland), England, using chironomid pupal exuviae', Spixiana, Supplement 14, pp. 167–74 [Online]. Available at www.landesmuseum.at/pdf_frei_remote/SpixSupp_014_0167-0174.pdf (Accessed eleven March 2013).
Acknowledgements
This course was written by Suresh Nesaratnam.
Except for third party materials and otherwise stated (see terms and conditions), this content is made available under a Creative Eatables Attribution-NonCommercial-ShareAlike 4.0 Licence.
The material acknowledged beneath is Proprietary and used under licence (non subject to Creative Commons Licence). Grateful acknowledgement is made to the following sources for permission to reproduce material in this grade
Grade paradigm: Kris Krug in Flickr fabricated available under Creative Commons Attribution-NonCommercial-NoDerivs 2.0 Licence.
Grateful acknowledgement is made to the following sources.
Figure ane: Cartoon by Ajit Nunan. Courtesy of the Centre for Science and Surroundings, New Delhi.
Effigy 3: Department of Geology and Geography, Ohio Wesleyan University.
Figures 7, 9 and ten: Copyright © CNRI/Science Photo Library.
Figures 8 and 11: Copyright © Eye of Scientific discipline/Science Photo Library.
Figure 12: Copyright © RGB Ventures LLC dba SuperStock/Alamy.
Every effort has been made to contact copyright owners. If any have been inadvertently overlooked, the publishers will exist pleased to brand the necessary arrangements at the first opportunity.
Don't miss out:
If reading this text has inspired you to learn more than, y'all may be interested in joining the millions of people who discover our gratuitous learning resource and qualifications by visiting The Open University - www.open.edu/ openlearn/ complimentary-courses
Copyright © 2016 The Open Academy
Source: https://www.open.edu/openlearn/ocw/mod/oucontent/view.php?id=18241&printable=1
Posted by: troupeheith1981.blogspot.com

0 Response to "Are Aquatic And Costal Systems Denialated More Clearly By Their Animal Life?"
Post a Comment